Approval of Elahere Expands Treatment Options for Some Advanced Ovarian Cancers
Updated: 2024-04-24 00:00:00
 On Monday, new recommendations from a panel of independent experts (NutriRECS https://nutrirecs.com/about/) say that most people can continue to consume red and processed meat at their average current consumption levels. We asked nutritional epidemiologist Marji McCullough, ScD RD, about the … Continue reading →
On Monday, new recommendations from a panel of independent experts (NutriRECS https://nutrirecs.com/about/) say that most people can continue to consume red and processed meat at their average current consumption levels. We asked nutritional epidemiologist Marji McCullough, ScD RD, about the … Continue reading →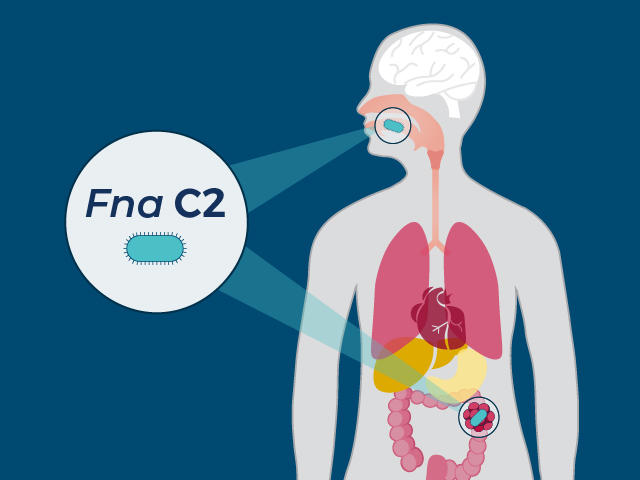 NCI-funded researchers have pinpointed a single type of the bacterium F. nucleatum that appears to fuel the development and growth of colorectal cancer. In mice, the bacterium, Fna C2, appeared to cause more adenomas to form in the large intestine and it was often found in human tumor samples.
NCI-funded researchers have pinpointed a single type of the bacterium F. nucleatum that appears to fuel the development and growth of colorectal cancer. In mice, the bacterium, Fna C2, appeared to cause more adenomas to form in the large intestine and it was often found in human tumor samples.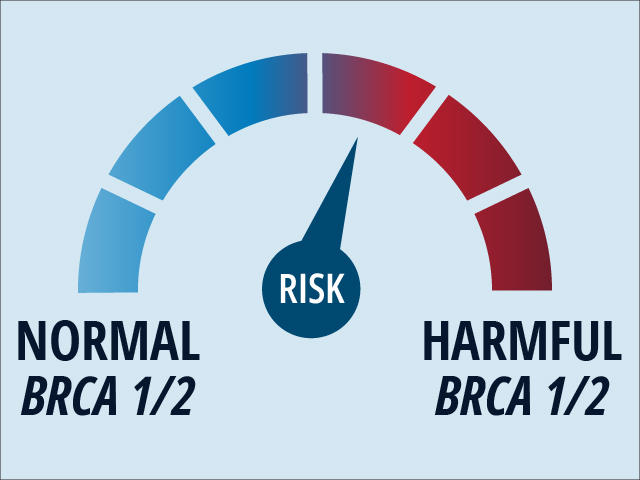 For women with inherited changes in the BRCA1 or BRCA2 genes, regular MRI scans and undergoing a surgery called a salpingo-oophorectomy appear to lower their chances of dying from breast and ovarian cancer, according to results from two studies.
For women with inherited changes in the BRCA1 or BRCA2 genes, regular MRI scans and undergoing a surgery called a salpingo-oophorectomy appear to lower their chances of dying from breast and ovarian cancer, according to results from two studies.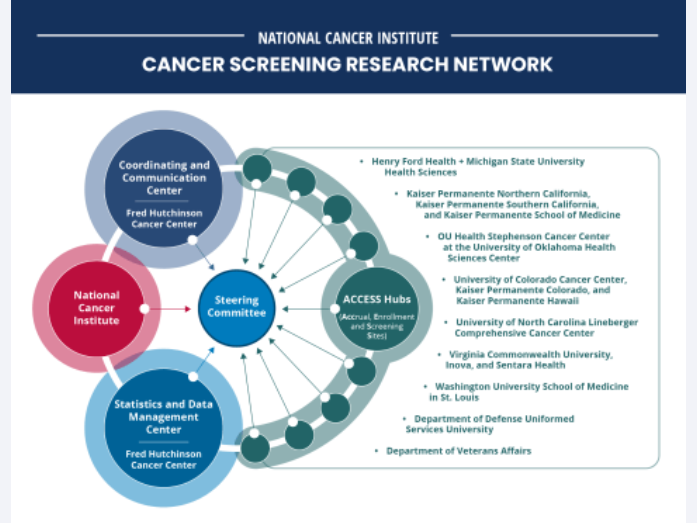 NCI periodically provides updates on new websites and other online content of interest to the cancer community. See selected content that has been added as of April 2024.
NCI periodically provides updates on new websites and other online content of interest to the cancer community. See selected content that has been added as of April 2024.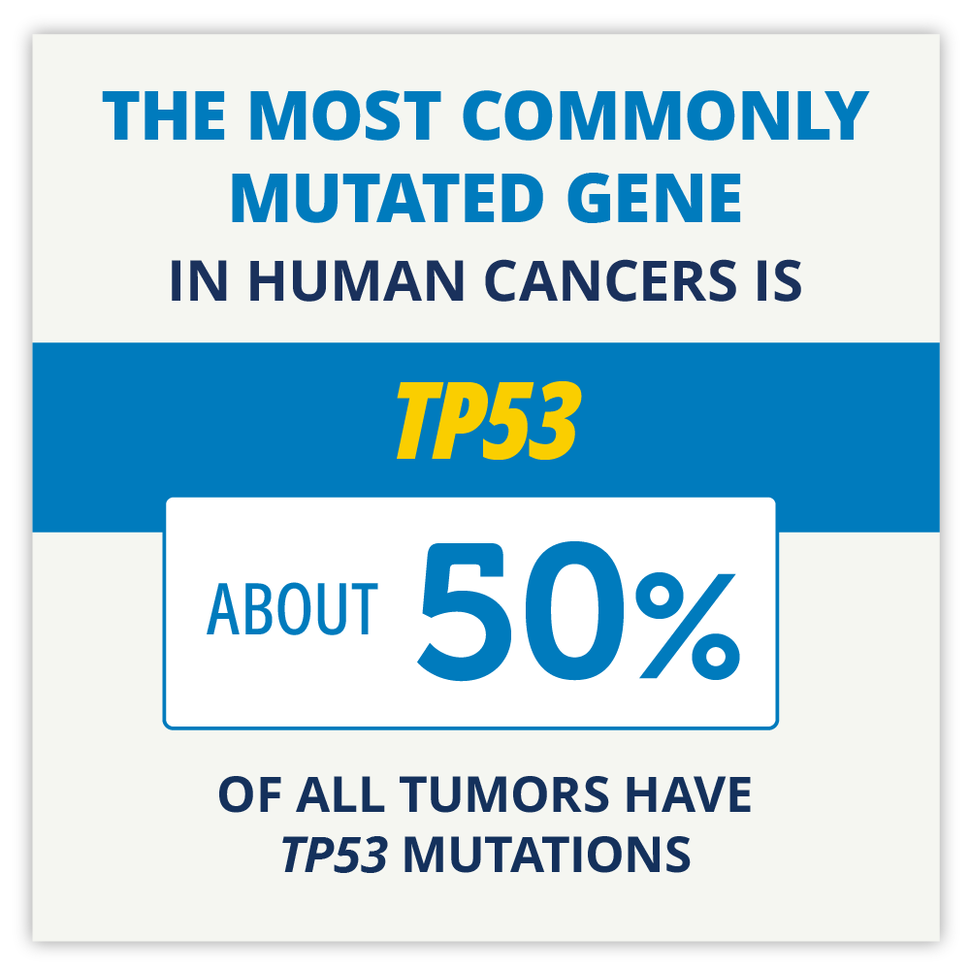 Although TP53 mutations help drive the growth of most cancers, there are no FDA-approved therapies that target altered p53 proteins. Now a drug combination has shown promise in mice and is being tested in a clinical trial.
Although TP53 mutations help drive the growth of most cancers, there are no FDA-approved therapies that target altered p53 proteins. Now a drug combination has shown promise in mice and is being tested in a clinical trial.